New Hampshire Backyard Aquaponics -- Preparations for Spring 2014
Greetings everyone,
I have an aquaponics thread I started on AR15.com in the summer of 2012. derek saw it over there and invited me over here a while back. Great site BTW.![Grin [grin] [grin]](/xen/styles/default/xenforo/smilies.vb/041.gif) My aquaponics thread is still ongoing over on AR15.com, but I am going to make some significant changes and I'd like to document them here for your enjoyment and education as well. There are likely others here who are also doing this and can both add to the discussion and provide constructive criticism.
My aquaponics thread is still ongoing over on AR15.com, but I am going to make some significant changes and I'd like to document them here for your enjoyment and education as well. There are likely others here who are also doing this and can both add to the discussion and provide constructive criticism.
First, a quick definition of aquaponics that I borrowed from here.
Aquaponics is the cultivation of fish and plants together in an constructed, re-circulating ecosystem using natural bacterial cycles to convert fish waste to plant nutrients. It's a natural food growing method that harnesses the best attributes of aquaculture and hydroponics without the need to waste any water or filtrate or add chemical fertilizers.
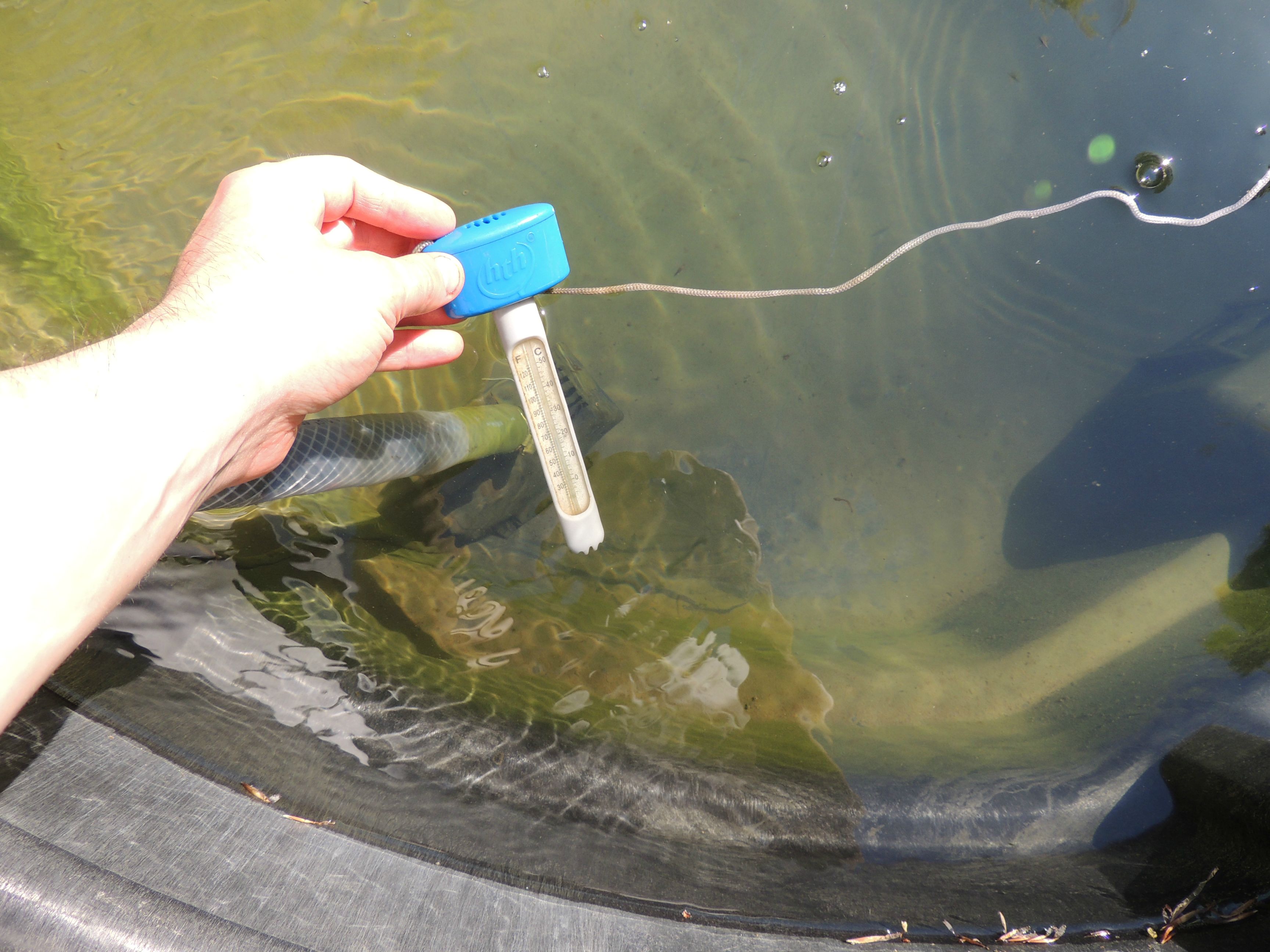
Aquaponics in the northeast is unique for several reasons including: wide fluctuations in temperature, the types of fish and plants that can be grown, and the fact that the system needs to be "shut down" during the winter due to freezing temperatures unless it is brought indoors or at least into a greenhouse and additional heat supplied. For my aquaponics system, I will not be bringing the fish indoors, but instead, if all goes well, will allow them to hibernate in near freezing water temperatures.
The system I started up in the summer of 2012 used mainly Brown Bullhead catfish since fingerling sized fish are easily caught throughout the northeast and the fish are notorious for their hardiness. This makes them very forgiving when conditions in the system go to extremes compared to other fish. Ironically, one of the other fish I have found to be almost bulletproof when it comes to water conditions are goldfish and more recently Yellow Perch.
When I originally started researching aquaponics in the summer of 2011, I really wanted to try the system with Channel Catfish but the two batches of fish I got from local pet stores were killed off by ich (protozoan parasite) as there was no way for me to treat them outdoors.
Last summer, I decided to try the Channel catfish again, but this time using an indoor aquarium type system to grow them to a size suitable for putting outdoors this spring. My first batch died when a power strip tripped and I lost filtration and aeration. So I bought another batch this past fall and started over again. Fortunately, I have made it to this point, and barring any other problems I'm going to put them outdoors in the next month or so.
I would like to go over some basic principles first. Let's start with a simple scenario where we have a bucket filled with water and some fish.

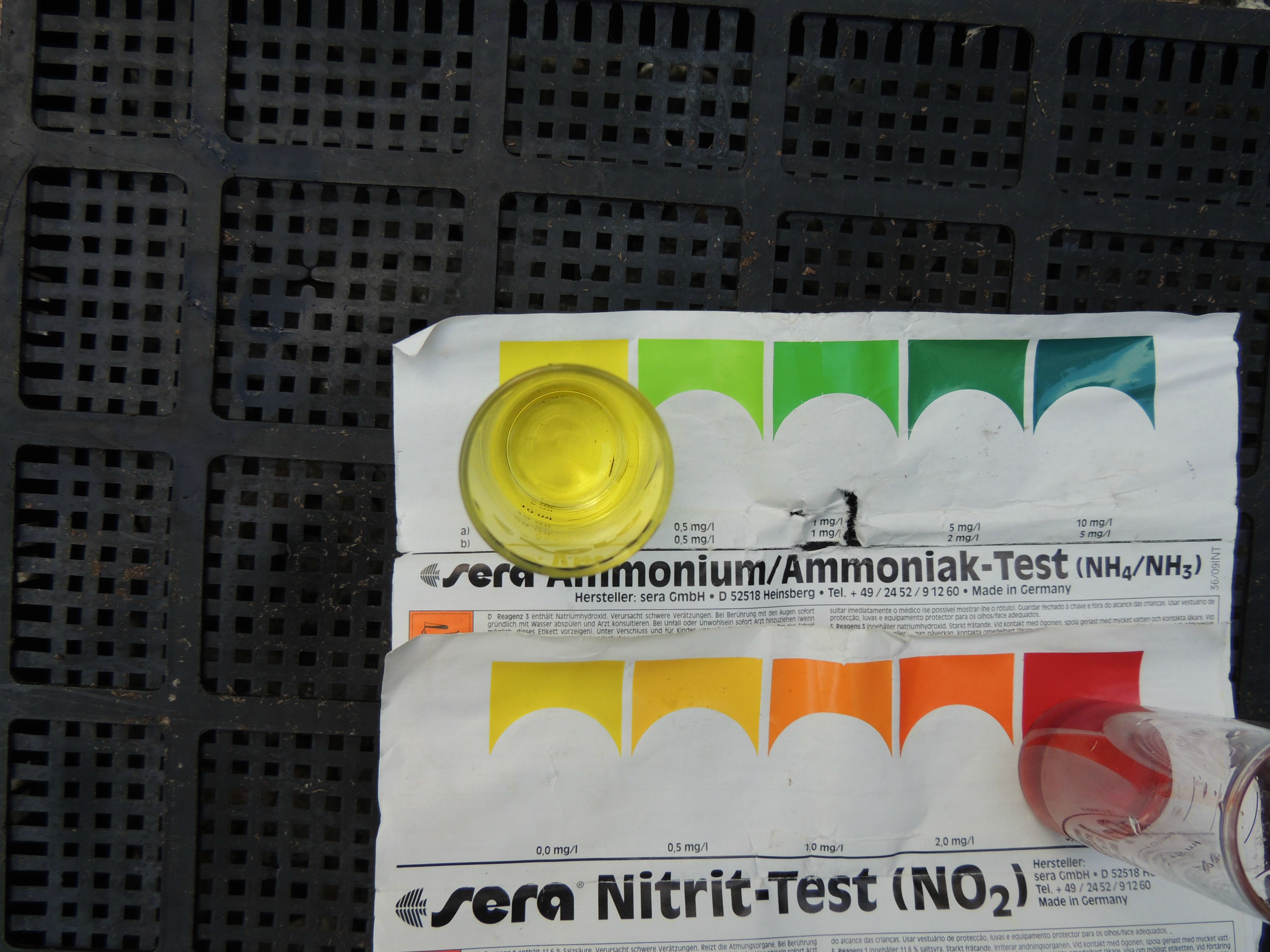
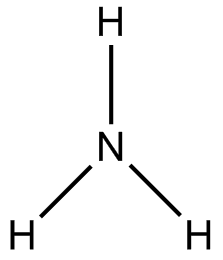
Fish, like many other animals, produce waste that they must excrete or rid from their body in some way. The most toxic and abundant of these wastes is ammonia, NH[SUB]3
[/SUB][SUB]I will discuss the source of the ammonia later. To lessen the toxicity of ammonia, many animals combine ammonia, with carbon dioxide using the Urea Cycle:
[/SUB]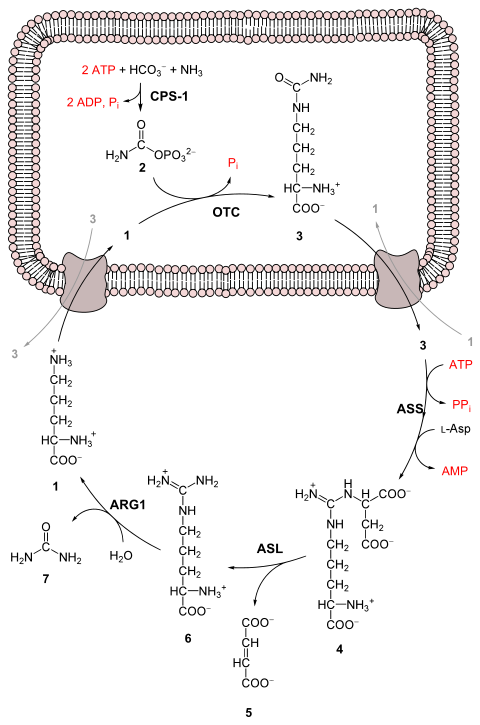 [SUB]
[SUB]
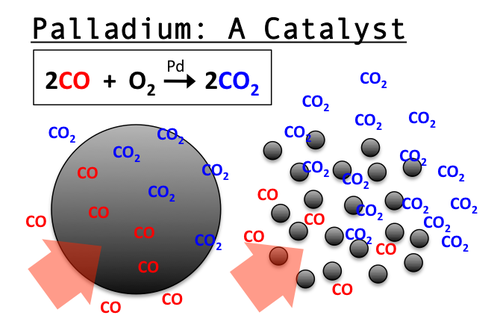
No, you don't have to memorize the above. This conversion does require energy on the part of the animal. The overall equation is as follows:
2NH[SUB]3[/SUB] + CO[SUB]2[/SUB] -----------------→ CO(NH[SUB]2[/SUB])[SUB]2[/SUB] + H[SUB]2[/SUB]O
ammonia + carbon dioxide ----→ urea + water
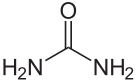
Urea molecule:
Why do animals convert ammonia (NH[SUB]3[/SUB]) into urea? Urea is much less toxic than ammonia, so it is a way for the body to transport ammonia through the bloodstream without causing a lot of toxicity. Once the urea reaches the kidneys, the latter filter the urea out of the blood Yes, fish do have kidneys that filter the urea from the blood. The kidney makes urine that travels to the bladder and from there the urea-containing urine is expelled into the surrounding water. If you've ever gutted a fish, you may have noticed the urinary bladder (as opposed to the air-filled swim bladder). During the stress of being on a hook, the fish often expels all the urine so it may not be full and therefore more difficult to see.
There is another method by which fish get rid of ammonia. Instead of combining it with carbon dioxide (CO[SUB]2[/SUB]) to make urea which then goes out through the bladder, ammonia can get expelled directly from the bloodstream into the surrounding water by diffusing across the fish's gills. The advantage of this method is the fish does not expend any energy as the ammonia crosses from the fish into the water as the concentration is higher in the blood than in the water around the fish.
Now back to our container of fish . . .
[/SUB][SUB]
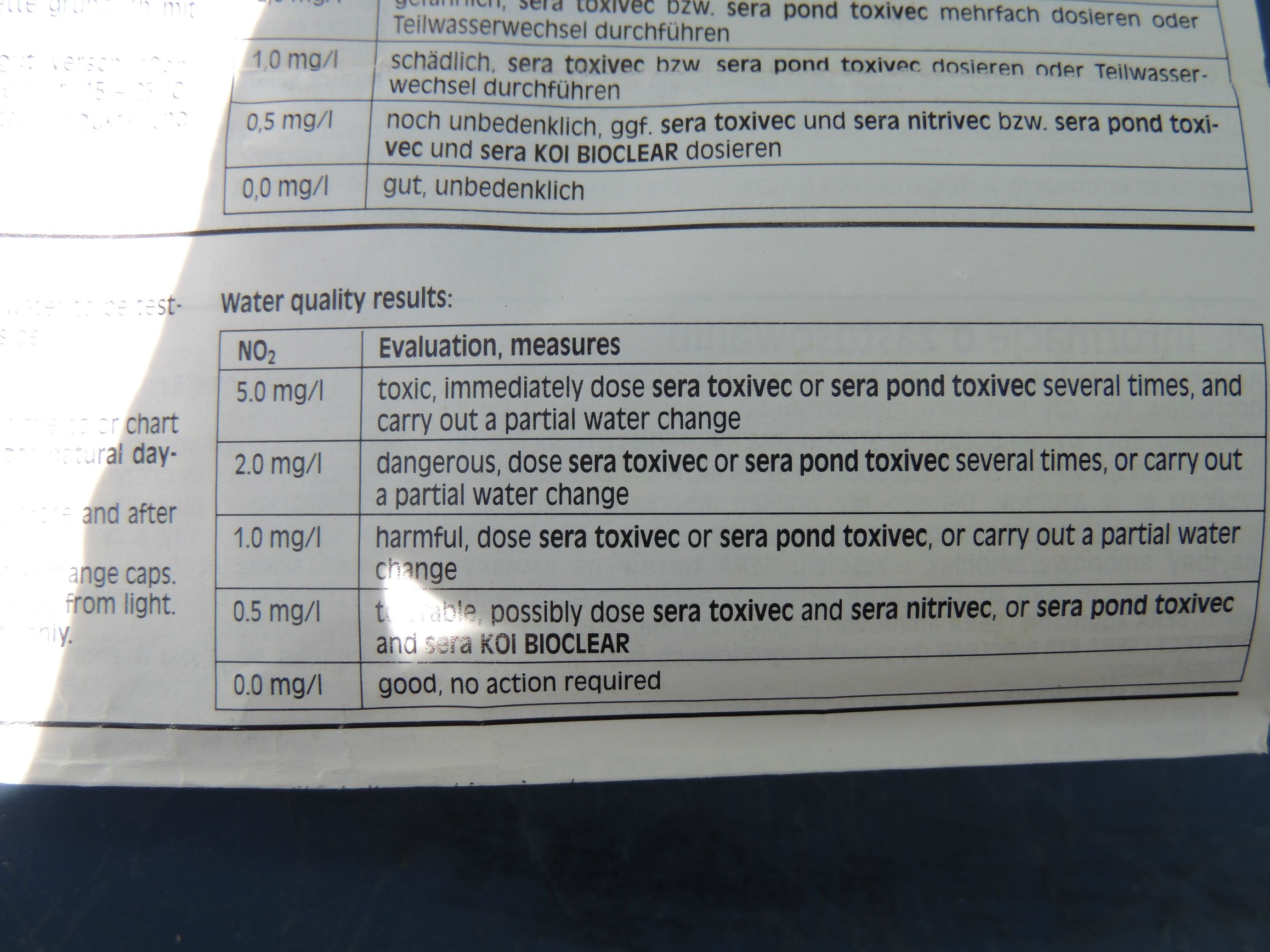
As the fish produce ammonia and urea waste, the concentration of these products begin to increase in the surrounding water. If fresh water is used to replace the ammonia and urea-laden water, then the ammonia never reaches a toxic concentration. Ideally, there should be zero ammonia as even small amounts can affect the fish's health. But if the amount of water exchanged keeps the ammonia levels low, the fish will remain healthy. If, however, the ammonia is allowed to build up, then eventually it will reach a lethal concentration. The fish are literally bathing in their own waste. Not to be graphic, but this would be like drinking your own urine instead of fresh, clean water. Interestingly, the fish will all die within about 24 hours of each other, that is how predictable lethal ammonia levels are.
A quick point about urea: Many bacteria have the enzyme urease which breaks urea back down into ammonia and carbon dioxide. Because of this, urea in a container of fish will get broken back down into ammonia. If you've ever left a toilet bowl full of urine overnight, this is the reason the toilet smells strongly of ammonia. Your bladder (and likely the toilet bowl) contain bacteria that will break urea back into ammonia, giving off that strong smell.
To summarize:
1) Fish produce ammonia as waste.
2) Ammonia is very toxic and is disposed of by the fish in the surrounding water.
3) If the ammonia is not removed from the water, the fish will eventually die.
Pretty simple so far. I will either edit this post or add another one. More to come.![Grin [grin] [grin]](/xen/styles/default/xenforo/smilies.vb/041.gif) [/SUB]
[/SUB]
Greetings everyone,
I have an aquaponics thread I started on AR15.com in the summer of 2012. derek saw it over there and invited me over here a while back. Great site BTW.
![Grin [grin] [grin]](/xen/styles/default/xenforo/smilies.vb/041.gif) My aquaponics thread is still ongoing over on AR15.com, but I am going to make some significant changes and I'd like to document them here for your enjoyment and education as well. There are likely others here who are also doing this and can both add to the discussion and provide constructive criticism.
My aquaponics thread is still ongoing over on AR15.com, but I am going to make some significant changes and I'd like to document them here for your enjoyment and education as well. There are likely others here who are also doing this and can both add to the discussion and provide constructive criticism.First, a quick definition of aquaponics that I borrowed from here.
Aquaponics is the cultivation of fish and plants together in an constructed, re-circulating ecosystem using natural bacterial cycles to convert fish waste to plant nutrients. It's a natural food growing method that harnesses the best attributes of aquaculture and hydroponics without the need to waste any water or filtrate or add chemical fertilizers.
Aquaponics in the northeast is unique for several reasons including: wide fluctuations in temperature, the types of fish and plants that can be grown, and the fact that the system needs to be "shut down" during the winter due to freezing temperatures unless it is brought indoors or at least into a greenhouse and additional heat supplied. For my aquaponics system, I will not be bringing the fish indoors, but instead, if all goes well, will allow them to hibernate in near freezing water temperatures.
The system I started up in the summer of 2012 used mainly Brown Bullhead catfish since fingerling sized fish are easily caught throughout the northeast and the fish are notorious for their hardiness. This makes them very forgiving when conditions in the system go to extremes compared to other fish. Ironically, one of the other fish I have found to be almost bulletproof when it comes to water conditions are goldfish and more recently Yellow Perch.
When I originally started researching aquaponics in the summer of 2011, I really wanted to try the system with Channel Catfish but the two batches of fish I got from local pet stores were killed off by ich (protozoan parasite) as there was no way for me to treat them outdoors.
Last summer, I decided to try the Channel catfish again, but this time using an indoor aquarium type system to grow them to a size suitable for putting outdoors this spring. My first batch died when a power strip tripped and I lost filtration and aeration. So I bought another batch this past fall and started over again. Fortunately, I have made it to this point, and barring any other problems I'm going to put them outdoors in the next month or so.
I would like to go over some basic principles first. Let's start with a simple scenario where we have a bucket filled with water and some fish.

Fish, like many other animals, produce waste that they must excrete or rid from their body in some way. The most toxic and abundant of these wastes is ammonia, NH[SUB]3

[/SUB][SUB]I will discuss the source of the ammonia later. To lessen the toxicity of ammonia, many animals combine ammonia, with carbon dioxide using the Urea Cycle:
[/SUB]
 [SUB]
[SUB]No, you don't have to memorize the above. This conversion does require energy on the part of the animal. The overall equation is as follows:
2NH[SUB]3[/SUB] + CO[SUB]2[/SUB] -----------------→ CO(NH[SUB]2[/SUB])[SUB]2[/SUB] + H[SUB]2[/SUB]O
ammonia + carbon dioxide ----→ urea + water
Urea molecule:

Why do animals convert ammonia (NH[SUB]3[/SUB]) into urea? Urea is much less toxic than ammonia, so it is a way for the body to transport ammonia through the bloodstream without causing a lot of toxicity. Once the urea reaches the kidneys, the latter filter the urea out of the blood Yes, fish do have kidneys that filter the urea from the blood. The kidney makes urine that travels to the bladder and from there the urea-containing urine is expelled into the surrounding water. If you've ever gutted a fish, you may have noticed the urinary bladder (as opposed to the air-filled swim bladder). During the stress of being on a hook, the fish often expels all the urine so it may not be full and therefore more difficult to see.
There is another method by which fish get rid of ammonia. Instead of combining it with carbon dioxide (CO[SUB]2[/SUB]) to make urea which then goes out through the bladder, ammonia can get expelled directly from the bloodstream into the surrounding water by diffusing across the fish's gills. The advantage of this method is the fish does not expend any energy as the ammonia crosses from the fish into the water as the concentration is higher in the blood than in the water around the fish.
Now back to our container of fish . . .
[/SUB][SUB]
As the fish produce ammonia and urea waste, the concentration of these products begin to increase in the surrounding water. If fresh water is used to replace the ammonia and urea-laden water, then the ammonia never reaches a toxic concentration. Ideally, there should be zero ammonia as even small amounts can affect the fish's health. But if the amount of water exchanged keeps the ammonia levels low, the fish will remain healthy. If, however, the ammonia is allowed to build up, then eventually it will reach a lethal concentration. The fish are literally bathing in their own waste. Not to be graphic, but this would be like drinking your own urine instead of fresh, clean water. Interestingly, the fish will all die within about 24 hours of each other, that is how predictable lethal ammonia levels are.
A quick point about urea: Many bacteria have the enzyme urease which breaks urea back down into ammonia and carbon dioxide. Because of this, urea in a container of fish will get broken back down into ammonia. If you've ever left a toilet bowl full of urine overnight, this is the reason the toilet smells strongly of ammonia. Your bladder (and likely the toilet bowl) contain bacteria that will break urea back into ammonia, giving off that strong smell.
To summarize:
1) Fish produce ammonia as waste.
2) Ammonia is very toxic and is disposed of by the fish in the surrounding water.
3) If the ammonia is not removed from the water, the fish will eventually die.
Pretty simple so far. I will either edit this post or add another one. More to come.
![Grin [grin] [grin]](/xen/styles/default/xenforo/smilies.vb/041.gif) [/SUB]
[/SUB]
Last edited:

![Smile [smile] [smile]](/xen/styles/default/xenforo/smilies.vb/001.gif) ).
). 








![Sad [sad] [sad]](/xen/styles/default/xenforo/smilies.vb/004.gif)

![Thinking [thinking] [thinking]](/xen/styles/default/xenforo/smilies.vb/010.gif)
![Wink [wink] [wink]](/xen/styles/default/xenforo/smilies.vb/002.gif) We'll get into cycling the system to grow bacteria. During this period, many people will not put plants in since you get big spikes in nitrite which can be toxic to plants. I've also read that the risks are overstated since plants can process nitrite to some extent. My plan this year is to maximize the growth of the best plant for my system so as to absorb as much nitrate as possible. That happens to be horseradish for my system. It has grown extremely well in the last two years for me. I will likely plant some cucumbers as well. Here is a picture of the horseradish that I've started indoors. I saved the entire root of one plant from the aquaponics system last year in a ziplock bag in the refrigerator. I cut inch-long pieces for planting. The leaves have grown out much more so. I will wash off the peat/soil before planting it in the expanded shale.
We'll get into cycling the system to grow bacteria. During this period, many people will not put plants in since you get big spikes in nitrite which can be toxic to plants. I've also read that the risks are overstated since plants can process nitrite to some extent. My plan this year is to maximize the growth of the best plant for my system so as to absorb as much nitrate as possible. That happens to be horseradish for my system. It has grown extremely well in the last two years for me. I will likely plant some cucumbers as well. Here is a picture of the horseradish that I've started indoors. I saved the entire root of one plant from the aquaponics system last year in a ziplock bag in the refrigerator. I cut inch-long pieces for planting. The leaves have grown out much more so. I will wash off the peat/soil before planting it in the expanded shale.